Abstract
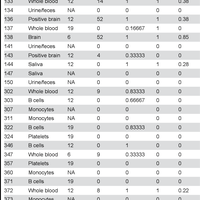
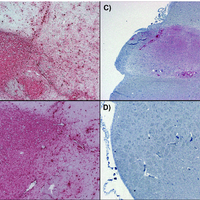
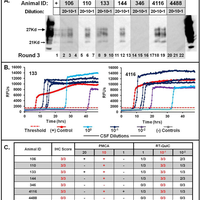
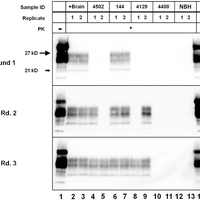
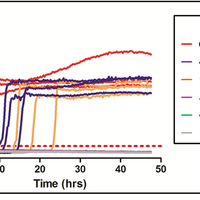
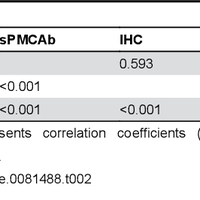
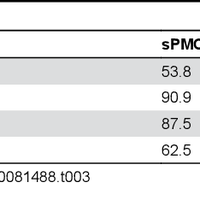
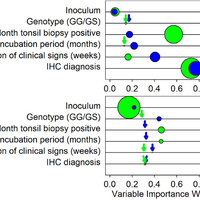
Transmissible spongiform encephalopathies (TSEs), or prion diseases, are a uniformly fatal family of neurodegenerative diseases in mammals that includes chronic wasting disease (CWD) of cervids. The early and ante-mortem identification of TSE-infected individuals using conventional western blotting or immunohistochemistry (IHC) has proven difficult, as the levels of infectious prions in readily obtainable samples, including blood and bodily fluids, are typically beyond the limits of detection. The development of amplification-based seeding assays has been instrumental in the detection of low levels of infectious prions in clinical samples. In the present study, we evaluated the cerebrospinal fluid (CSF) of CWD-exposed (n=44) and naïve (n=4) deer (n=48 total) for CWD prions (PrPd) using two amplification assays: serial protein misfolding cyclic amplification with polytetrafluoroethylene beads (sPMCAb) and real-time quaking induced conversion (RT-QuIC) employing a truncated Syrian hamster recombinant protein substrate. Samples were evaluated blindly in parallel with appropriate positive and negative controls. Results from amplification assays were compared to one another and to obex immunohistochemistry, and were correlated to available clinical histories including CWD inoculum source (e.g. saliva, blood), genotype, survival period, and duration of clinical signs. We found that both sPMCAb and RT-QuIC were capable of amplifying CWD prions from cervid CSF, and results correlated well with one another. Prion seeding activity in either assay was observed in approximately 50% of deer with PrPd detected by IHC in the obex region of the brain. Important predictors of amplification included duration of clinical signs and time of first tonsil biopsy positive results, and ultimately the levels of PrPd identified in the obex by IHC. Based on our findings, we expect that both sPMCAb and RT-QuIC may prove to be useful detection assays for the detection of prions in CSF. © 2013 Haley et al.
Figures
Register to see more suggestions
Mendeley helps you to discover research relevant for your work.
Cite
CITATION STYLE
Haley, N. J., Van De Motter, A., Carver, S., Henderson, D., Davenport, K., Seelig, D. M., … Hoover, E. (2013). Prion-seeding activity in cerebrospinal fluid of deer with chronic wasting disease. PLoS ONE, 8(11). https://doi.org/10.1371/journal.pone.0081488